Overview
| Top |
|
BioBanking Accessioning Data Entry |
Overview |
|
|
The Sample Accession page is used to collect and modify additional information about the Sample prior to Approval, and to conduct the Approval itself. This page is accessed by clicking the "Accession Samples " button on any of these Sample List pages:
| • | Allocated Samples |
| • | Data Entry Samples |
| • | Lab Operations Samples and Child Samples |
| • | Admin Samples |
| • | Accessioning Pane of the Receive and Accession Samples Task |
In any of the Sample List pages, select one or more Samples, then click "Accession Samples". The Sample Accession page displays:
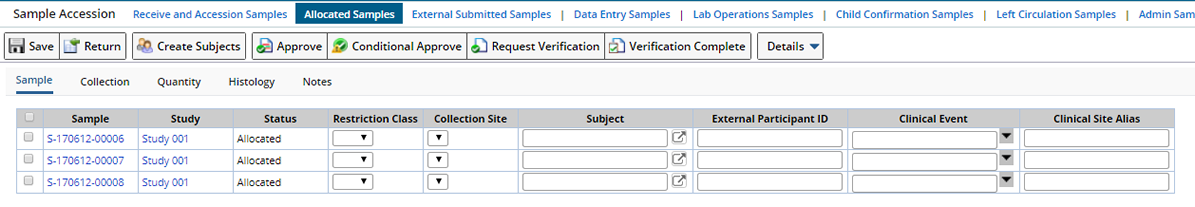
When multiple Samples are selected, clicking the Sample Id hyperlink (or the word "Sample" when only one Sample is selected) opens the View Sample page for that Sample.
| NOTE: | Depending on the type of Study (Protocol or Non-Protocol) different fields display during Accessioning. When selecting multiple Samples all must be within the same type of Study. |
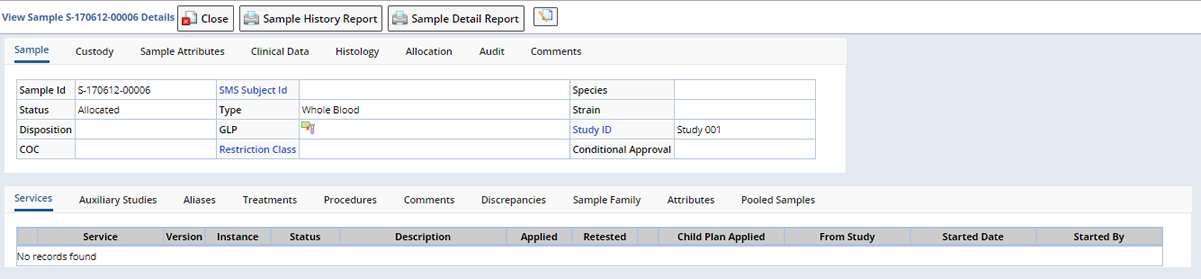
Accessioning Data |
|
|
Information to collect is grouped by category. Provide information about the Sample itself, how it was collected and in what quantities, as well as the Histology of the Sample.
Sample |
The Sample tab displays demographic information about the Sample. Depending on whether the Study is a Non-Protocol or Protocol Study, different fields display in the Sample Tab.
Non-Protocol Accessioning
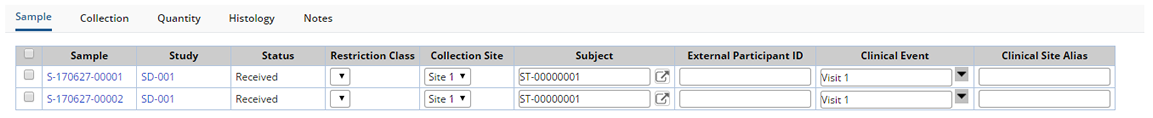
Protocol Accessioning

| Field | Description |
| Sample
Study |
The Sample being Accessioned. The Study for which the Sample is being Accessioned is also listed. Click the Study id to view details about the Study. |
| Status | The Sample Status. |
| Status | The Status of the Sample. |
| Restriction Class | The Restriction Class associated with the Sample. See also Consent Questions for ways to manage consent. |
| Collection Site | Site from which the Sample was collected. |
| Subject (Non-Protocol only) | The Subject from whom the Sample was received. |
| Cohort (Protocol only) | The Cohort with which the Sample donor is associated. |
| External Participant ID | External identifier used by the Site to identify this Subject in this Study. |
| Clinical Event (Non-Protocol only) | The associated Clinical Events. Choose the Events for which the Samples were collected. |
| Visit> Timepoint (Protocol only) | The defined (in the Study) Visit and Timepoint at which the Sample was collected. |
| Clinical Site Alias | Define a Clinical Site Alias. |
Collection |
Annotate the Sample with additional information about the Sample itself and collection details.
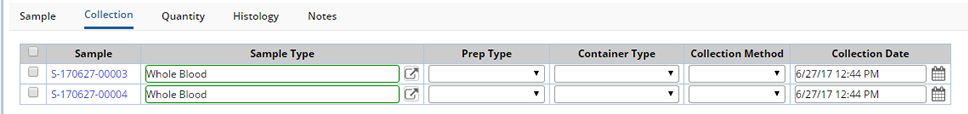
| Field | Description |
| Sample | The Sample Id and Study for which the Sample is being Received. |
| Sample Type | The Type of Sample being Received. Choose a Sample Type from the dropdown. |
| Prep Type | Identify any Sample Preparation. Choose a Preparation Type from the dropdown. |
| Container Type | The Type of Container Received. Choose a Container Type from the dropdown. |
| Collection Method | Identify a method for Sample Collection. Choose a Collection Method from the dropdown. |
| Collection Date | The date on which the Sample was collected. |
Quantity |
Detail Sample Quantities and Concentration.
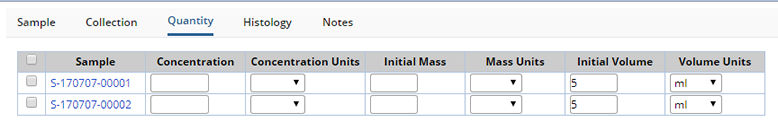
| Field | Description |
| Concentration Concentration Units |
This is the Concentration of the Sample. |
| Init Mass Init Mass Units |
For solid tissues, this is the mass of the Sample. |
| Init Volume Init Volume Units |
For liquid Samples this is how much of Sample. |
Histology |
Detail Sample Quantities and Concentration.
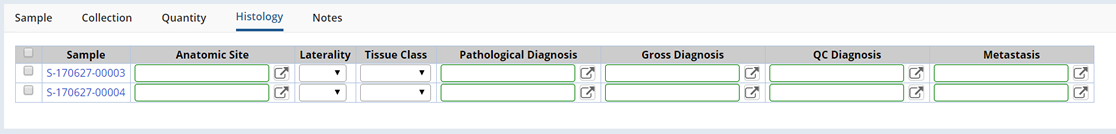
| Field | Description |
| Anatomic Site | Specify the type of Tissue received. Choose a Tissue Type from the Tissue Tree lookup. |
| Laterality | Specify Left or Right Laterality. Available values are defined in the Laterality Reference Type. |
| Tissue Class | Specify the Tissue Class (such as Normal or Abnormal). Available values are defined in the Tissue Class Reference Type. |
| Pathalogical Diagnosis | Choose a Microscopic Diagnosis from the lookup. |
| Gross Diagnosis | Choose a Microscopic Diagnosis from the lookup. |
| QC Diagnosis | Choose a Microscopic Diagnosis from the lookup. |
| Metastasis | Define a Metastasis. Choose a Metastasis from the lookup. |
When you are finished entering additional information click "Save".
Accessioning Data Entry Operations |
|
|
These are the buttons at the top of the Accessioning Data Entry pages (see the Receive and Accession Samples Task for a list of operations available within the task):
| Operation | Description | Visible on these pages... |
| Create Subjects | This option appears only while performing accession data entry on Non-Protocol study samples. This button facilitates automatic
population of data entry fields.
Suppose no matching Subjects are found in the BioBanking system for the selected Samples. In this case, "Create Subject" creates Subjects for the selected Samples if all of the Subjects share the same attributes. A Subject form appears after clicking the button: After populating the fields in this form, clicking "Save & Assign" creates one new Subject for each selected Sample. The Subject form closes, and the Accessioning Data Entry Grid refreshes with the newly created Subjects shown in the grid. |
Accessioning Data Entry Grid |
| Approve | Changes Sample status to "In
Circulation" after the system verifies that all information entered for
the Sample has met the approval requirements defined in the Study.
For both "Conditional Approve" and "Approve", if all required information has been entered for the Samples, the storage status changes to "In Circulation". If validation fails, a page appears with specific violations found: |
Accessioning
Data
Entry Grid
Accessioning Data Entry Form |
| Conditional Approve | Approves Samples (putting them into circulation) for the user with the Conditional Approval flag set, thereby allowing full approval (see "Approve") when all required information has been supplied. | Accessioning
Data
Entry Grid
Accessioning Data Entry Form |
| Request Verification | Sets Sample status to "Verification Needed". | Accessioning
Data
Entry Grid
Accessioning Data Entry Form |
| Verification Complete | Completes the verification process and resets Sample status to "In Circulation". | Accessioning
Data
Entry Grid
Accessioning Data Entry Form |