Overview
| Top |
|
BioBanking Studies |
Content |
||||||||||||||||||
|
Overview |
|
|
A Study is a concept that uniquely groups a collection of Samples under a specific experimental premise or clinical Protocol. Generally studies are a set of Samples collected loosely from different sources that are to be used for a common purpose such as general discovery science. Each Sample is permanently associated with one Study.
LabVantage provides two types of Studies:
| • | Protocol Studies Protocol Studies allow the definition of versioned Protocols that can specify a series of Sample collection Events with associated Assays to apply to the Samples (requires BioBanking Protocol Management Module). |
| • | Non-Protocol Studies Non-Protocol Studies allow the definition of specific Clinical Events for which Samples can be collected. |
Study design can include any of the following:
| • | Assign a Contact to the Study. |
| • | Clinical Sites for Non-Protocol Studies to identify where Samples are collected. |
| • | Sites to identify the different Sites from which Samples are collected for a Protocol Study. |
| • | Versioned Protocols (Protocol Studies). |
| • | Define Clinical Events for Non Protocol Studies. |
| • | Services are the processes, procedures and tests to be performed for the Study. |
| • | Choose to require Approvals before a Study can be initiated. |
| • | Approval Rules to specify the minimum information required of a Sample before it can be used in the laboratory (InCirculation). |
| • | User Document Access to define LabVantage Users assigned particular access to the documents of a Study. |
| • | Forms that can be used to collect information. |
| • | Restriction Classes to identify Samples whose use is restricted. |
| • | Consent Questions to obtain consent for a Samples use. In the case of Non-Protocol studies, Consent Questions are defined in the study. In the case of Protocol Studies, the Consent Questions are defined per revision. |
Create a New Study |
|
|
Before adding a new Study you may need to define Study Template(s).
Note: Whether or not a Template must be referenced when adding a new Study is determined in Biobanking Policy.
Study Template |
Study Templates allow you to pre-define a typical Study, then reference the Template to default all the information defined here to a new Study. To add a Study Template navigate to Lab Admin → Templates → Study Templates. The Study Template List page displays. Click "Add".
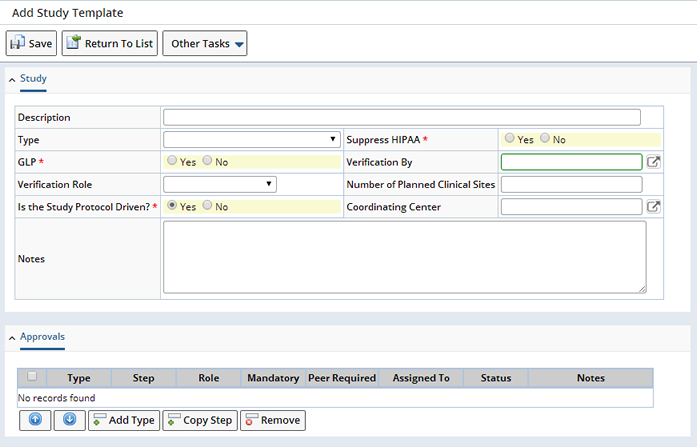
All the details of a Study can be pre-defined in the Template, you can make changes to the defaulted information at the Study level. See Design the Study for detailed information about the fields on the Add Study Template page. Define general information about the Study including whether or not the Study is a Protocol or Non-Protocol driven Study. "Save" the information, a prompt for the Study Template Identifier is provided. Enter the Template ID and click "OK".
After saving, the detail tabs display:
Once Templates are defined you can Add a new Study by navigating to LIMS → Biobanking → New Studies. The Study List page displays. Click "Add". If prompted, select a Study Template and/or enter the Study Id for the new Study.
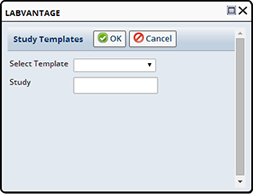
If you are not prompted for these initial values the Study Id must be entered on the Study Maintenance page.
Click "OK" to continue to the Study Maintenance page.
Design the Study |
Whether designing a Study as a Template or adding a new Study directly you are presented with the Study Maintenance page.
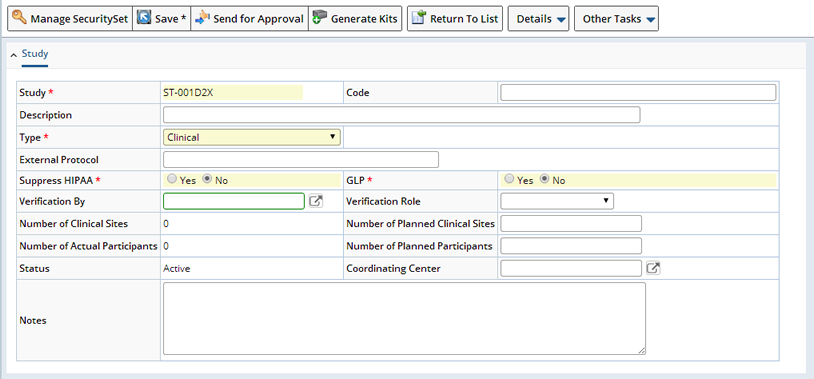
| Field | Description | ||||||||||||||
| Study | Unique identifier for a Study. This is copied to each Sample of the Study. Accordingly, this identifier can be configured as part of each individual Sample Id. | ||||||||||||||
| Code | Free text for internal Study Code designation. Must be unique across all Studies in the system. | ||||||||||||||
| Description | Free text description of Study (up to 80 characters). | ||||||||||||||
| Type | Enumerated list of Study Types.
This is a largely cosmetic field used to group Studies in reports.
Read-only if the Study is based on a template.
Two Study Types affect behavior of the system: Clinical types may require the next column External Protocol Id to be supplied and this depends on the "Study Has Protocol" rule in the BioBanking Policy. External Submitted types are used to permit Samples to be shown on a specialized List page for recording of externally submitted Samples. |
||||||||||||||
| External Protocol | Identifier of the external Clinical Protocol associated with the Study. Required if the Study Type is "Clinical" and the BioBanking Policy "Study Has Protocol" rule is active. | ||||||||||||||
| Suppress HIPAA | Indicates whether or not saving
the date of birth (DOB) during subject data entry would save the full
DOB or only the year (when set).
This flag also prevents the linking of any subject that bears a day and month of birth to Samples in this Study. If this flag is changed during the progress of the Study, it will apply only to Samples that have their data entry completed after the flag is set. |
||||||||||||||
| GLP | Indicates a new Sample's GLP flag for Samples belonging to this Study. During all allocation pathways, when a Sample is allocated in a Study with GLP set, Samples will default to GLP being set. | ||||||||||||||
| Verification By | This person has the ability to modify Sample data for Samples in this Study, regardless of their Role. This person is also the only specific person that can perform the additional verification on data entry (See Verification Role). If blank, no individual user is designated with this ability. | ||||||||||||||
| Verification Role |
The role of the users that have the ability to modify Sample data for Samples in this Study. Users with this role can also perform the additional verification on data entry. If blank, no role is designated with this ability. | ||||||||||||||
| # of Clinical Sites | Number of actual clinical sites currently associated with this Study (See Clinical Sites or Sites). Read-only. | ||||||||||||||
| # of Planned Clinical Sites | Number of sites planned to be part of this Study. | ||||||||||||||
| # of Actual Participants | Number of actual Subjects currently assigned as Participants in this Study. Read-only. | ||||||||||||||
| # of Planned Participants | Number of participants planned to be part of this Study. | ||||||||||||||
| Status | Current Status of a Protocol Study.
|
||||||||||||||
| Notes | Any additional annotations about this Study (up to 2000 characters). | ||||||||||||||
| Coordinating Center | Shipping location that is the coordinating center for the Study. This value is purely informational and has no special system behavior impact. |
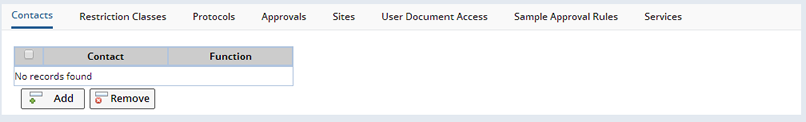
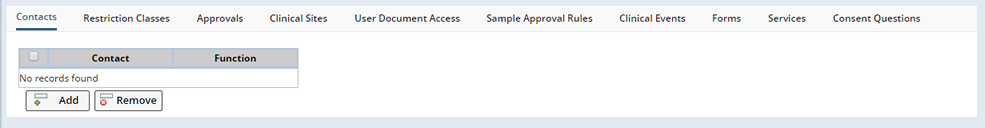
The detail tabs that display are determined by whether you are defining a Protocol or Non-Protocol Study.
Protocol Studies
Non-Protocol Studies
All detail tabs are described in the following sections.
Contacts |
As part of the Study definition, you can specify both internal and external Contacts that are related to the Study. This is purely informational (tells who can be contacted about the Study), and has no functional behavior associated with these Contacts.
The list of Contacts is shown as a separate tab on the Study maintenance page. For each Contact you specify, you must indicate the function of that Contact person.

Restriction Classes |
|
When Samples are collected as part of clinical Studies, registries, or license, there is typically a "consent" agreement that applies to the use of each Sample. Restriction Classes (RC) are used to represent these various "consent restrictions" and track them for every Sample. Tracking of the consent that applies to the Sample, and the chain of custody for the Sample, helps fulfill the legal responsibility to the patient who donated the materials.
Any Sample with a Restriction Class having the "COC Required" flag set must follow all COC procedures and record such procedures in order to preserve the Sample's exact location at all times.
Note: Depending on the configured BioBanking policies, no Sample can be received or approved for a Study unless the Sample is associated with at least one active Restriction Class. The system does not automatically create a Restriction Class for each Study.
There are several "basic" categories of restrictions that can apply to a Sample:
| • | Unrestricted These Samples are generally available for discovery and have no consent restrictions. They can be handled by any lab at any time without any special procedures, unless they are being used for GLP assays. |
| • | Consent-Restricted Samples These must be tracked in relation to the specific consent form to which they apply. They are typically collected in Studies or at research hospitals. Consent-restricted Samples, and their derivatives and aliquots, must have their physical custody constantly tracked (in the event consent is ever revoked). Their use may be restricted according to specific conditions in the consent form. Such restrictions may be broad ("any Oncology research"), or specific ("can only sequence gene FOX129"). |
| • | Business Agreement Restriction This is a usage restriction that applies to a specific agreement an organization has with another organization. Some restrictions require full chain of custody, others do not. |
To represent these various restrictions, each Sample maintains a "Restriction Class" field that indicates the Restriction Class to which it belongs. Each Study defines one or more Study-specific Restriction Classes to model the restrictions on the Study's Samples. Each Restriction Class describes the limitations that must be followed for Samples assigned to it. Restriction Classes are recorded purely as a human decision support advisory. Human operators will read the Restriction Class for a Sample and ultimately make the decision as to how to enforce the requirements set forth in the Restriction Class. During Sample data entry, a Restriction Class from the Sample's Study is assigned to the Sample. When an aliquot or derivative of a Sample is created, the Restriction Class of the child Sample is the same as that of the parent.
From the Study Maintenance page, Restriction Classes Tab, the "Add Restriction Class" button opens the add Restriction Class maintenance page shown below:
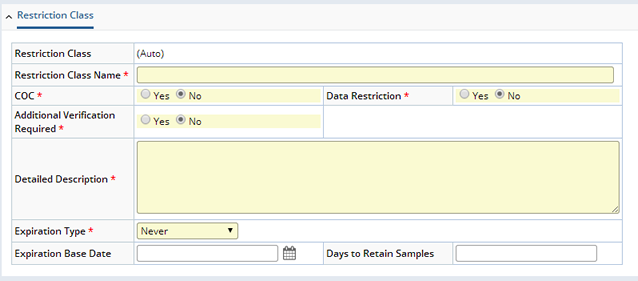
| Field | Description |
| Restriction Class | Automatically assigned identifier for the Restriction Class. The Study Id is part of this identifier. |
| Restriction Class Name | User's unique name for the Restriction Class. This is what is used throughout the system to view and select Restriction Classes. |
| COC | Indicates Chain of Custody procedures are applicable for Samples assigned this Restriction Class. |
| Data Restriction | Indicates data restrictions are applicable for Samples assigned this Restriction Class. |
| Additional Verification Required | Indicates if additional verification of Samples is required during data entry/approval. This value can be set only if the "Verification By" field of the Study is supplied. |
| Detailed Description | Free text detailed description of the meaning of the consent level (use, retention, distribution restrictions) for this Restriction Class. |
| Expiration Type | Enumerated list of expiration types (ExpirationType). The default is "Never". |
| Expiration Base Date | Base date for expiration of this Restriction Class and its associated Samples. |
| Days to Retain Samples | Number of days after Expiration base date the Sample can be retained. |
After saving the Restriction Class you can optionally add another Restriction Class by clicking "Add Another" or "Close and Refresh". Restriction Classes display in the Restriction Classes tab on the Study maintenance page:
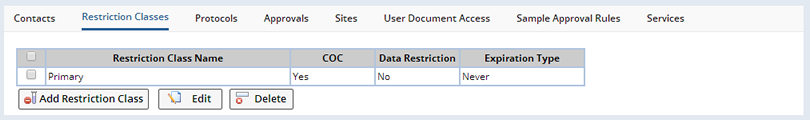
From this tab, you can choose any Restriction Class to edit or delete. When editing the Restriction Class, the previous Maintenance page is shown.
Protocols |
When creating a Protocol Study the Protocol detail tab displays. See Biobanking Protocols for detailed information about defining a Protocol Study.
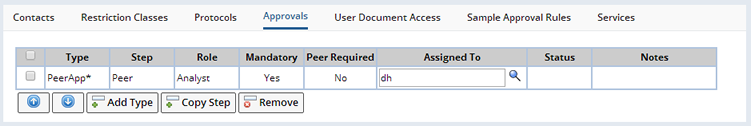
Approvals |
Depending on the requirements of a Study, it may require an approval process before it can become active. The necessary steps for approval can be added or modified from the Approvals tab on the Study Maintenance page:
Clinical Sites (Non-Protocol Studies Only) |
When full Protocol Studies are either not required or not available, Clinical Sites allows the definition of sites that apply to a particular Study. Each site is simply identified by a name. Operations are available to create, edit or remove sites. Sites that have been linked to existing Samples can be deactivated (by un checking the Active checkbox) if they are no longer applicable to the Study. This will remove the Clinical Site from any lists associated with the Study. Clinical Sites linked to Samples cannot be deleted.
As part of the Study Maintenance page, there is a tab for managing Clinical Sites:
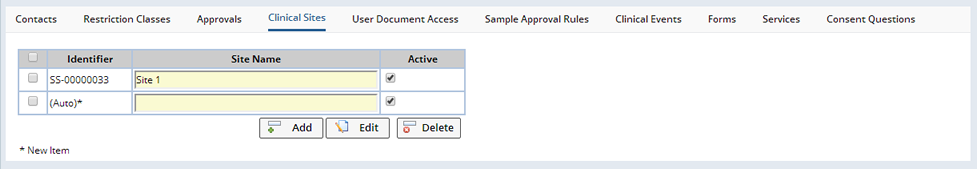
Sites (Protocol Studies Only) |
The system supports the ability to manage a set of collection Sites that apply to a particular Study. Each site is identified by a name and can be associated with an existing Custodial Department or Shipping Location (Department field). Operations are available to create, edit or remove Sites. Sites that have been linked to existing Samples can be deactivated, by un checking the Active checkbox, if they are no longer collecting Samples. This will remove the Site from any lists associated with the Study. Sites linked to Samples cannot be deleted.
As part of the Study Maintenance page, there is a tab for managing Sites:
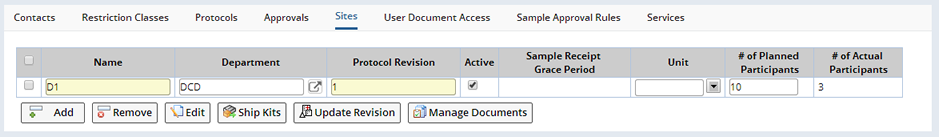
Editing a Site opens the Study Site Maintenance page and allows you to specify further attributes for the Site as well as define Contacts and Users for the Site. Site Contacts and Users function the same way and are managed the same way as Study Contacts and Users. From the Study Site Maintenance page it is also possible to list the Study Participants currently assigned to the Site.
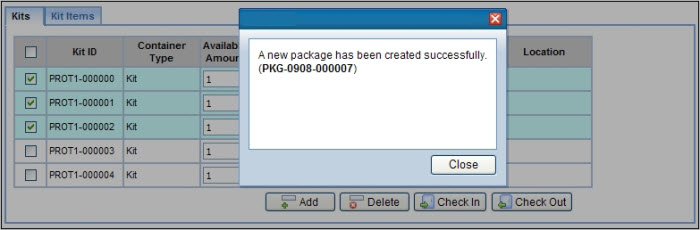
The Site tab allows selected Kits to be shipped to specific Sites. Upon clicking the "Ship Kits" button, a Kits lookup dialog opens allowing you to select the Kits and how many to be shipped to the Site. This creates a Package ready for shipment to the Site with the destination already set to the selected Site. Only those Kits defined for the current Study from Generate Kits, or valid generic Kits that have not yet been shipped, can be selected for shipment to a Site.
For Protocol Studies only, each Site must also specify which Protocol revision is being used for the Site. This allows different Sites to be using variations of the Protocol within the same Study. A Site's current Protocol revision can be updated using the "Update Revision" button. A Grace Period for Sample receipt may also be defined for Protocol Studies if there is a requirement for Samples to be received within a strict time period after collection.
If new Documents are required for a specific Site, or existing Documents need to be edited, reviewed or submitted, use the "Manage Documents" button to open the Documents management page for the selected Site.
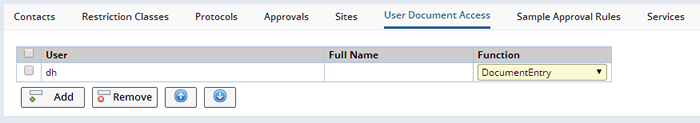
User Document Access |
Identify Study Users (LabVantage Users) that are assigned particular access capabilities to the Documents in the Study. These capabilities include document viewing, editing, approval and management. The User Document Access tab is used to manage these Users and their document capabilities:
Sample Approval Rules |
Defined in the BioBanking Policy are a series of rules that specify the minimum information required for a Sample before it can be used in the laboratory. This policy definition is used as the default rules for Sample approval in each Study, but can also be modified to suit the specific requirements of a Study. This editing is done in the Approval Rules tab:
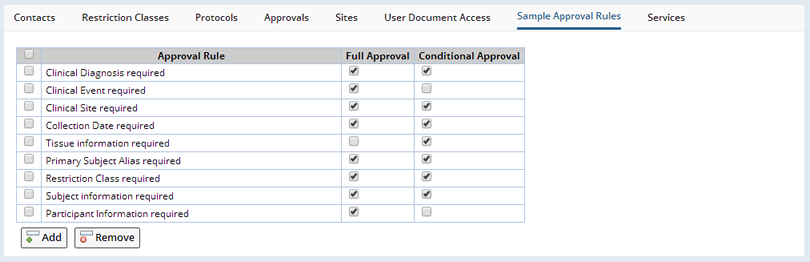
Add or remove Approval Rules and specify at which approval level (Full or Conditional Approval) each is required to approve the Sample.
Once a Sample is Approved it can be used and processed in the laboratory. Aliquots and Derivatives can be created, and the Sample can be shipped to different locations.
The only difference between Conditional Approved and Full Approved Samples is that Conditional Approved Samples are indicated by an icon in the Sample list pages to tell the User that further information is still required for the Sample before it can be fully Approved.
All of the required data values can be entered during Accessioning Data Entry, submission and processing of Sample Collection Forms, or possibly on the TISM page during Sample receipt.
The BioBanking Policy is initially configured with the following Approval Rules:| Approval Rule | Description | |||
| Anatomic Site required | Sample must be assigned an Anatomic Site to be Approved. | |||
| Clinical Diagnosis required | Sample must be assigned a Clinical Diagnosis to be
Approved.
| |||
| Clinical Event required | Sample must be assigned an Event to be Approved. Can apply for both Protocol driven Studies and regular BioBanking Studies. See Clinical Events and Protocol Events. | |||
| Clinical Site required | Sample must be assigned a Site to be Approved. See Clinical Sites or Sites. | |||
| Collection Date required | Sample must be assigned a Collection Date to be Approved. | |||
| Gross Diagnosis | Sample must be assigned a Gross Diagnosis (defined as part of the Biobanking Master Data Setup) to be Approved. | |||
| Laterality required | Sample must be assigned Laterality (Reference Type value) to be Approved. | |||
| Tissue Class required | Sample must be assigned a Tissue Class (Reference Type value) to be Approved. | |||
| Pathological Diagnosis required | Sample must be assigned a Pathalogical Diagnosis to be Approved (defined as part of the Biobanking Master Data Setup). | |||
| Primary Subject Alias required | Sample must be assigned an external Subject alias to be approved. If the External Participant is entered on the TISM page then the value is set as the external Subject alias for the Sample. | |||
| Subject information required | Sample must be assigned a Subject to be approved. If the External Participant is entered on the TISM page then the value is used to create a new Participant and Subject or match an existing Participant and Subject that are then assigned to the Sample. | |||
| Participant Information required | (Protocol Studies Only) Sample must be assigned a Participant, Visit and Protocol Revision to be approved. If the External Participant is entered on the TISM page then the value is used to create a new Participant or match an existing Participant that is then assigned to the Sample. | |||
| QC Diagnosis required | Sample must be assigned a QC Diagnosis (defined as part of the Biobanking Master Data Setup) to be Approved. |
Clinical Events (Non_Protocol Studies Only) |
When full Protocol Studies are either not required or not available, Clinical Events still allows the definition of specific collection timepoints during the course Study, albeit as only very simple timepoint names. From the Clinical Events tab it is possible to define multiple events and provide a name for each event:
During the accessioning data entry process for Samples it is possible to select a specific collection Clinical Event for the Sample from the Study's Clinical Event list, or even provide a new user defined timepoint name (although this will not be added to the Study's Clinical Events list). This is a much simplified and flexible definition of Clinical Events than that provided in Protocol Studies.
Forms (Non_Protocol Studies Only) |
During the course of a Study certain documents are completed by persons involved in the Study. Examples of such documents are Subject enrollment details, Subject consent information, Site material transfer agreements (MTA), details of Samples collected, etc. LabVantage has the capability to define templates of these documents as online Forms (See Forms) that can be completed to create Documents associated with particular SDIs. In the Study Maintenance page, in the Forms tab, it is possible to specify the Forms used by a particular Study:
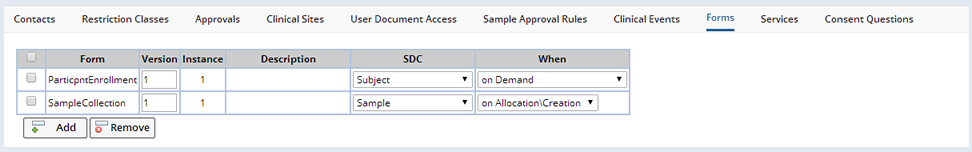
Once a Form is added, you can specify to which SDC Documents from this Form, will be associated as well as when such Documents are created. Selecting "on Allocation/Creation" for a Sample means that when a Sample is created for this Study, a blank Document based on the appropriate Form will be generated and attached to the new Sample. Selecting "on Association of 1st Sample" for a Subject means that when the first Sample from this Study is linked to a particular Subject, a blank Document based on the appropriate Form will be generated and attached to the Subject. Subsequent links of Samples to the same Subject for this Study will not generate new Documents, whereas Samples linked to the same Subject for a different Study will generate new Documents as the when rule is Study specific. Any blank Documents can then be completed by the user and saved. Documents can also be created "on Demand" by the user from from the Documents tab of various maintenance pages (the Forms lookup when the "Add" button is clicked is filtered by the list provided here as well as the relevant SDC), or from the Documents management page. When automatically creating blank Documents, if the Version field is left blank, then the latest active version of the specified Form will be used, otherwise any version of the specified Form can be used when created on demand.
If the SDC field is left blank, then this indicates that the Form is to be made available within the context of the Study Documents management page, accessed from the "Manage Documents" button on the Study List or Maintenance pages. This allows the definition of a short list of Forms specific to the Study.
Services |
Biobanking Services represent any action, process or testing that needs to be done to a biospecimen. Use Services to define Laboratory operations that may be performed on incoming samples.

When a Sample is created for a particular Study, these Services are automatically applied to the Samples as defined in the Services tab. These Services are not only applied to initially collected Samples but are also applied to child derivative Samples created for the Study.
When adding Services to Protocol Studies, in addition to the Services defined here, you can define Services within the Protocol, for each collected biospecimen individually. The Services are only applied to collected Samples for that Protocol. Specify a Sample Type so that only Samples created of that Sample Type will have the specified Service applied. See Services for detailed information about defining a Service.
Consent Questions |
Consent Questions allows Biobanking to determine consent, granted or denied, by Study Participants regarding the current and future use of donated Samples. Consent Questions can be defined at the following levels:
| • | Non Protocol Studies |
| • | Protocol Studies, at each individual Protocol Revision |
| • | Protocol Studies, at the Cohort level. Specify Cohort specific questions that override questions defined at the Protocol level. |
When defining Non-Protocol Studies, the Consent Questions tab displays in the detail tabs of the Study.
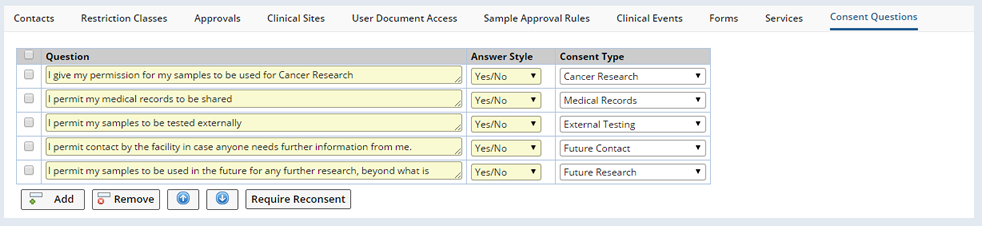
Click "Add" to add new questions.
| Field | Description |
| Question | Question to be asked of Participants. |
| Answer style | Choose Yes/No or Yes/No/NA (not applicable). |
| Consent Type | Reference Type defining the type of Consent needed. |
The Require Reconsent button adds the new or modified question to any Participant records containing this question (who are Enrolled this Study). Whenever an existing question requires Reconsent or a new question is added, all the exisiting Participants consent records need to be updated. Select the relevant questions and click "Require Reconsent". A dialog displays confirming the update.

The status of any previously answered questions for the associated Participants are set to "Pending". The status of new questions are also set to "Pending". The over all Consent status is updated as well.
When defining Protocol Studies Consent Questions are defined in the Protocol Revision.
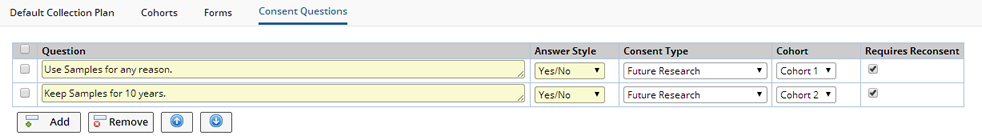
Click "Add" to add new questions.
| Field | Description |
| Question | Question to be asked of Participants. |
| Answer style | Choose Yes/No or Yes/No/NA (not applicable) |
| Consent Type | Reference Type defining the type of Consent provided. Defining a Consent Type enables you to Search for Samples by Consent Type. |
| Cohort | Define different Consent Questions for each Cohort. If no Cohort is specified all Participants will receive the question. |
| Requires Reconsent | Check to require Participants to Reconsent to changed or added questions. When versioning and uprevisioning the Protocol, the Consent Questions are copied to the new version/revision. The Consent Questions can be changed for each individual Protocol Revision. "Requires Reconsent" indicates that Reconsent is required when a new revision is created and Participants are uprevisioned. |
Consent Questions are answered from the Subject or Participant List, or Maintenance pages. See Enter Consent for more information.
Throughout Sample Management you have the opportunity to review answers to Consent Questions associated with a Subject Sample. In the various Sample list pages a column identifies the Consent Status of the Sample. Icons provide a visual cue as to the availability of the Samples. Consent is ![]() Granted or
Granted or ![]() Restricted. Consent is
Restricted. Consent is ![]() Pending until all questions are answered. If at any point a question is answered negatively (even if all questions have not yet been answered) Consent is Restricted.
Pending until all questions are answered. If at any point a question is answered negatively (even if all questions have not yet been answered) Consent is Restricted.
![]()
Clicking the icon opens the Participant Consent dialog.
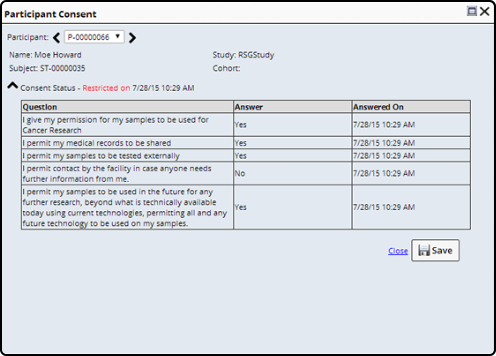
Here you can view the answers to the specific questions asked, and determine whether or not the Restriction applies to your need.
As questions are added or changed on a Study, and a Reconsent is requested, the Subject is updated to include the changes. All previous versions of the Consent Questions display.
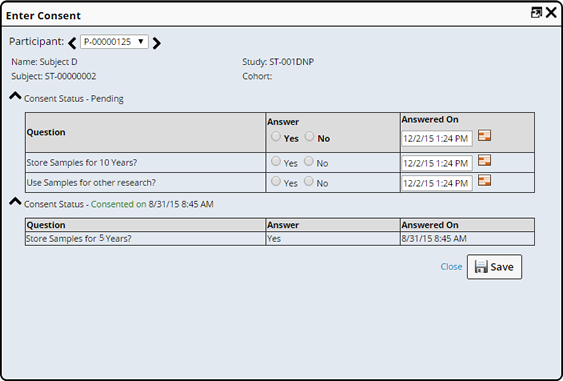
Study Maintenance |
| |
Once a Study is added its Status determines on which List page it appears. From the List Pages you can perform additional functions or maintenance.
Study List Pages |
Study functionality is distributed across multiple Study List pages, accessed from the LIMS → BioBanking menu and based on the state of the Studies as described below:
| List Page | Use | Available Operations |
| New Studies | Shows new Studies that have just
been created. This page shows only Studies that are not yet approved or active. | Add, Edit, Cancel, Generate Kits, Manage Documents |
| Studies for Approval | Used to approve Studies. Shows Studies that are pending approval. | Review, Manage Documents |
| Active Studies | Shows Studies that are currently
active. | Add, Edit, Complete, Cancel, Generate Kits, Manage Documents |
| Historical Studies | Shows Studies that have been completed or canceled and are no longer active. | View, UnCancel, UnComlete (in the Lifecycle menu) |
Following is a description of each operation:
| Operation | Description |
| Add | Opens the Study Maintenance Page
to allow the creation of a new Study. |
| Edit | Opens the Study Maintenance Page to allow the modification of the selected Study. |
| Review | Opens the Study Maintenance Page to allow the review of the selected Study before approval. |
| View | Opens the Study Maintenance Page in read-only mode to view the selected Study. |
| Copy | Makes a copy of an existing Biobanking Study. This copies
both Protocol and Non Protocol Studies. All details are copied, including
Protocol revisions, Services and embedded Child Sample Plans. |
| Delete | Deletes the selected Study. |
| Complete | Sets the status of the selected
Study to "Completed" and makes the Study in-active. |
| UnComplete | If the selected Study is completed
in error this operation returns it to its previous state. Requires an
E-Signature. |
| Cancel | Sets the status of the selected Study to "Canceled" and makes the Study in-active. |
| UnCancel | If the selected Study is canceled in error this operation returns it to its previous state. Requires an E-Signature. |
| Manage Documents | Opens the Documents management page for the selected Study. From this page documents can be added, edited, viewed, submitted and reviewed for the Study. Only those Forms that are defined for the Study in the Study Forms or Protocol Forms tabs can be added as new Documents. |
| Generate Kits | Opens the Kit Type Lookup Page to
allow the creation of Kits that have been defined for the selected
Study. |
Study Approval |
| |
In order for Approvals to be completed, the Study must first be "Submitted for Approval" from the Study Maintenance page. Once submitted for approval the Study is no longer available for editing from the New Studies list page, but must be accessed from the Studies for Approval list page for review and approval. Upon review of a Study it can be approved or rejected using the Approvals dialog and a subsequent e-signature:
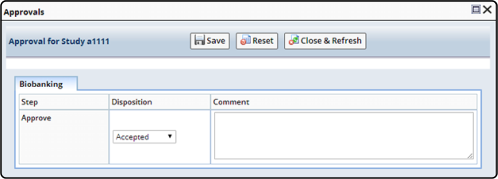
When a Study is approved it is then made active and accessible from the Active Studies list page. If a Study is rejected it returns to an editable state and is accessible from the New Studies list page.
If a Study is created from a template that does not contain any Approval Types, then the Study is immediately marked as Active and accessible from the Active Studies list page. Such Studies are not submitted for approval and bypass any approval step.
Generate Kits |
| |
From both the Study list and maintenance pages it is possible to create the Kits required for the Study. This is available for both protocol and non-protocol Studies.
The "Generate Kits" button, on the Study list and maintenance pages, opens a Kit Type lookup page to select the type of Kits to generate. By default this lookup displays the Kit Types used for the different events defined in a protocol Study, but the search bar and queries can be used to list other Kit Types.
From the Kit Type lookup page it is possible to create a new Kit Lot containing the specified number of Kits. Kits generated in this way are associated directly with the Study and the Kit Ids typically contain the Study Code. Using the "Generate Kits" button, on the Kit Type lookup page, you will be prompted for the number of Kits to generate and then be shown a Kit Lot Maintenance page:
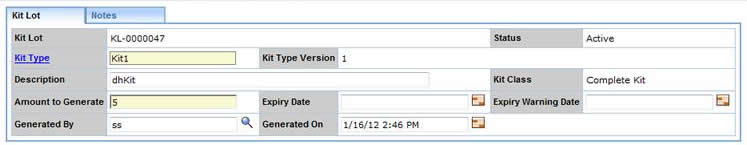
On this page further details of the Kit Lot can be entered and saved. Once the Kit Lot is created, pressing the "Create Shipment" button will initiate a Package containing the selected Kits that can then be shipped to any of the Sites defined for the Study: