Overview
| Top |
|
BioBanking Subjects |
Content |
||||||||||||
|
Overview |
|
|
A Subject is used to store basic information related to the donor of a Sample. Every Biobanking Sample (except Pooled Samples) in the system must be connected to a single "Subject" that represents the organism that produced the material. The concept of a Subject is used to model patients, animals, cell lines, and xenografts (any case where any biological tissue from one organism is added to another organism).
Defining Subjects gives you the opportunity to identify restrictions on Samples donated by the Subject, such as HIPPA suppression or negative answers to Consent Questions defined in the Study. Choose to add Subjects manually, ahead of Sample Receipt, or automatically during Sample Receipt.
When a Subject is enrolled in a Study a Participant record is created detailing the Subject's participation in the Study. The Participant record represents the Study/Subject relationship.
Protocol Study Enrollment
When enrolled in Protocol Studies, Participants are assigned to one of the Enrolling Study Sites as well as a particular Revision of a Study Protocol and to a specific Cohort. The assignment of the Study Protocol Revision is a result of choosing one Enrolling Study Site over another. Each Site is associated to one Revision. Therefore Participants are Enrolled to a particular Revision according to the Enrolling Study Site.
Non Protocol Study Enrollment
When enrolled in Non Protocol Studies, the Participant is also a record of the Subjects's enrollment into a Study. These Participants are created in two ways.
| • | Manually The Participant is enrolled in the Study the same way a Participant enrolls in a Protocol Study, however, there is no selection of a Cohort, or any Protocol Revision reference. |
| • | Automatically Behind the scenes, on the receipt of the first Sample for this Subject for this Study, the Participant association is created. |
The following diagram illustrates the relationships between Subjects and Participants.
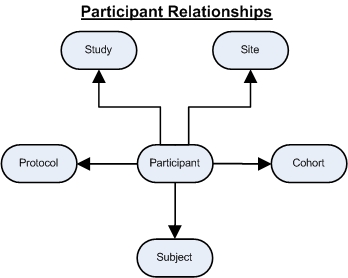 |
There are two ways in which Participants and Subjects are handled in the system.
| • | Subjects and Participants are Separate Entities Specific Subjects are enrolled into Studies to create a Participant record. This allows the same Subject to be enrolled as a Participant in multiple Studies. It also allows important attributes to be assigned to the appropriate Subject or Participant record. |
| • | Subjects are Created for Every New Participant The system automatically creates a new Subject for every new Participant. This requires the Auto Create Subject policy to be set. In this situation there is a one-to-one relationship between Subject and Participant and does not allow the same Subject to be a Participant in multiple Studies. |
Subjects |
|
|
Subjects can be entered either manually, or automatically during Sample Receipt.
Adding a Subject |
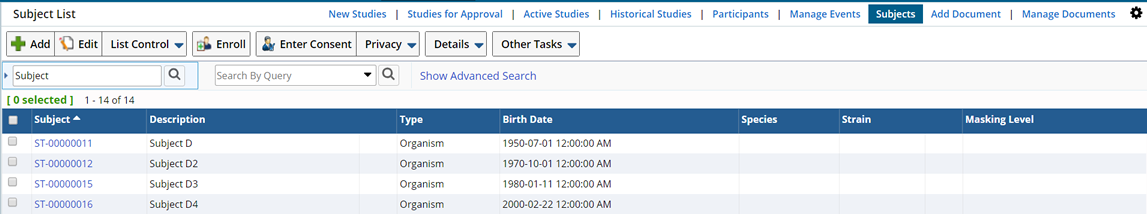
To add a new Subject navigate to LIMS → Biobanking → Subjects. The Subject List Page displays.
|
Following is a list of the Operations and tasks you can perform from the Subject List Page.
| Button | Description |
| Add | Adds a new Subject manually. |
| Edit | Edit existing Subjects. |
| Enroll | Enrolls Subjects into a Study. |
| Enter Consent | Answer the Consent Questions defined in the Study, into which the Subject is being Enrolled. See Enter Consent for detailed information about Consent Questions. |
| Privacy
Personal Info. Report Anonymize |
Choose to print a Privacy Info. Report or permanently remove (or anonymizes) private User data. See Data Privacy for more information. |
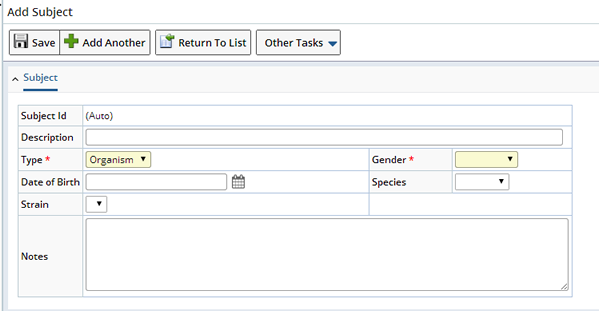
Choosing "Add" displays the Add Subject page.
|
| Field | Description |
| Subject Id | Unique identifier of the Subject, automatically generated. |
| Description | Text description of the Subject (up to 80 characters). |
| Type | Types of Subjects that can be represented (Organism, Xenograft, Cell Line). |
| Gender | List of genders (Male, Female, Unknown). |
| MRN | Include the Subject's Medical Record Number (MRN). |
| Date of Birth | Birth date of the Subject if applicable. |
| Date of Death | Death date of the Subject if applicable. |
| Species | List of Species. See Defining Species for more information about how this list is populated. |
| Strain | List of Strains. Users see only strains for species they select. |
| Notes | Additional annotations about this Subject (up to 2000 characters). |
Enter information about the Subject and "Save". Upon saving the detail tabs display.
Aliases |
Each Subject in the system supports multiple Subject Aliases. Subjects may be identified differently at each site, or for different external Protocols.
LabVantage also supports Aliases against the Participant record. Participant Aliases are useful when Studies have their own identifiers for Participants. These would be better modeled as Participant Alias, then the Subject Aliases.
In some cases, when Protocol Studies are not available or used, the system's record for a Subject may still correspond closely to a patient enrolled in a study, and the system must connect the contributed Samples to records within an external clinical database. A composite value created by concatenation of the optional external Protocol Id, Clinical Site Id, and external Patient Id is stored as a Subject Alias. This value corresponds to the Clinical Data Management System's (CDMS) unique key, referred to as a "Clinical Subject ID" (CSI). This is used to assist data managers in selecting the appropriate Subject to associate with a Sample.
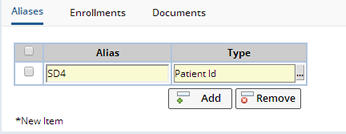
| Field | Description |
| Alias | Alias assigned to this Subject. |
| Type | Type of Alias. |
Enrollments |
The Enrollments tab shows, in read-only mode, any Participant records for the Subject. This details the Subject's enrollment in Studies:
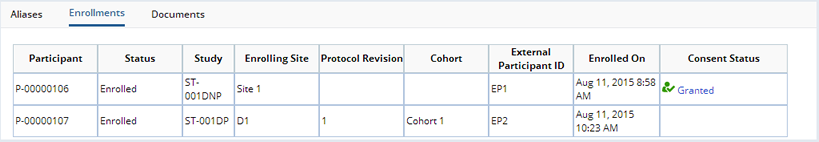
| Field | Description | ||||||||
| Participant | AutoKey-generated identifier of the Participant. | ||||||||
| Status | Current status of the Participant in the Study. One of "Enrolled", "Completed", or "Withdrawn". | ||||||||
| Study | Study in which the Subject is enrolled as a Participant. | ||||||||
| Enrolling Site | Site to which the Participant
belongs for Sample collection for the Study.
|
||||||||
| Protocol Revision | Protocol revision used for the Participant's Sample collection. | ||||||||
| Cohort | Cohort in the Protocol to which the Participant belongs. | ||||||||
| External Participant ID | External identifier used by the Site to identify this Subject in this Study. | ||||||||
| Enrolled On | Date on which the Subject was enrolled in the Study. | ||||||||
| Consent Status | The status of a donor's Consent based on answers to Consent Questions defined in the Study.
|
Documents |
From the Documents tab, new Documents can be added and existing Documents can be managed. Only Documents based on Forms defined in the Protocol as being allowed to be associated with a Subject can be created from this tab.
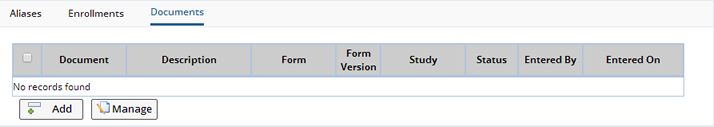
Upon clicking the "Add" button, the Documents management page opens where you can select a new Form for completion and submission.
If an existing Document needs to be edited, reviewed or managed, select the document and click the "Manage" button.
The ability to view, add, edit and manage documents in the Subject Maintenance page is controlled by the user's role in the Subject's Study as defined in the Study Users tab of the Study Maintenance page.
Enroll Subjects into a Study |
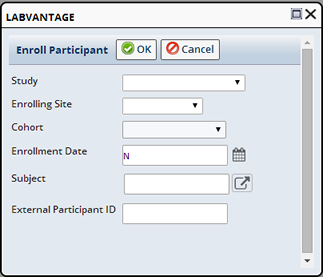
Using the Enroll Button on the Subject List page, select one or more Studies and click "Enroll". The Enroll Participant dialog displays.
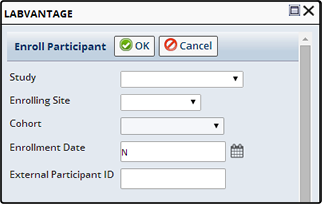
| Field | Description |
| Study | Choose the Study in which to Enroll the Subject. |
| Enrolling Site | The Site into which to Enroll the Subject. |
| Cohort | Choose to enroll the Subject into a specific Cohort. Cohort is disabled for Non-Protocol Studies. |
| Enrollment Date | The date the Subject was enrolled. |
| External Participant ID | External identifier used by the Site to identify this Subject in this Study. |
Click "OK". A Participant record is automatically created and the Subject is enrolled in the specified Study.
Enter Consent |
Subjects enrolled in a Study may need to answer Consent Questions defined within the Study. Clicking "Enter Consent" displays the Enter Consent dialog.
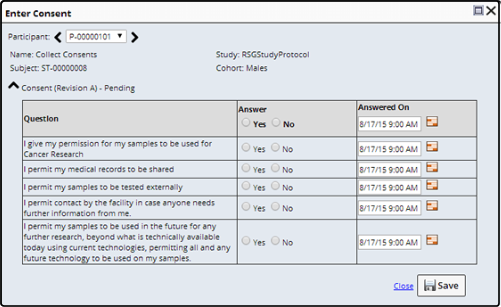
Answer the questions and specify the date on which the questions were answered. "Save" the answers.
Depending on the answers, Samples associated with this Subject or Participant are marked as either consent Granted ![]() or Restricted
or Restricted ![]() . Consent is Restricted when any question is answered negatively.
. Consent is Restricted when any question is answered negatively.
Consent is Pending ![]() until all questions are answered. Should an answered question create a Restriction, before all the questions are answered, the status changes to Restricted.
until all questions are answered. Should an answered question create a Restriction, before all the questions are answered, the status changes to Restricted.
Adding a Subject at the time of Sample Receipt |
While using the Receive and Accession Samples task you have the opportunity to Add a new Subject if a matching Subject is not found. See Receive and Accession Samples for more information.
Study Participants |
|
|
A Participant is a Subject who is enrolled into a study (Study/Subject relationship). The Participant has an initial status of "Enrolled" that may be subsequently changed to "Completed" once the Participant has completed the Study or Protocol, or to "Withdrawn" if the Participant withdraws from the Study. The following diagram illustrates the lifecycle of a Participant.
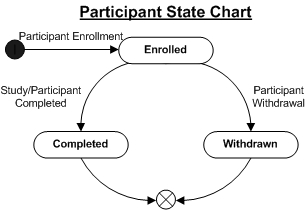
When a Child Sample is created from a Biobanking Sample associated with one Study, and the Child Sample is then associated with a different Study, a Participant record is created with the status "Associated".
| NOTE: | OOB, a single Participant record is created when a unique Study/Subject relationship is defined (when a Subject is enrolled into a Study) regardless of Site. For backward compatibility, if you prefer, you can optionally create a Participant record each time a Subject interacts with a different Site (associated with the Study) creating multiple Participant records for the Subject within the Study. See the "Participant Creation Rule" in the Biobanking Policy for more information. |
Enrolling Participants |
To enroll a Subject as a Participant in a Study select one or more Subjects on the Subject List page and click "Enroll". You can also enroll Subjects from the Participant List page, using the "Add/Enroll" button (this creates a new Participant record). A Subject may only be enrolled (as a Participant) once in a Study.
Provide the following details:
A Participant record is created and automatically enrolled in the current Protocol revision for the selected Study and Cohort.
Aditionally, Participants (or Subjects) can be automatically enrolled in a Study in any of the following ways:
| • | BioBanking Policy
The Auto Create Subject option in the BioBanking Policy determines whether or not to automatically create a Subject when a new Participant record is added. |
| • | Form Processing Use Form Processing to enroll Participants in a Study. |
| • | TISM Page Based on information provided during Sample receipt in the TISM page. |
In such cases a new Subject is also created for the Participant providing a 1 to 1 relationship between Subject and Participant.
If an online Form is to be used to enroll Participants, the eForm Manager page can be accessed from the "Manage Documents" button on the Study List or Maintenance pages. From this context only Study specific enrollment Forms can be selected. Alternatively the Documents management page can be opened from the "Add Document" menu item to see all online Forms. On the first occurrence of a Sample from this Participant, the new Participant will be Enrolled.
From the Documents management page a new Enrollment Form can be selected:
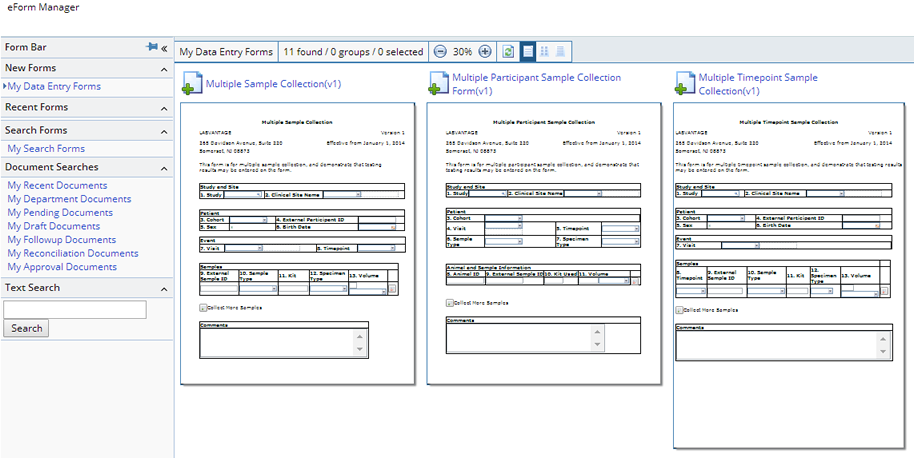
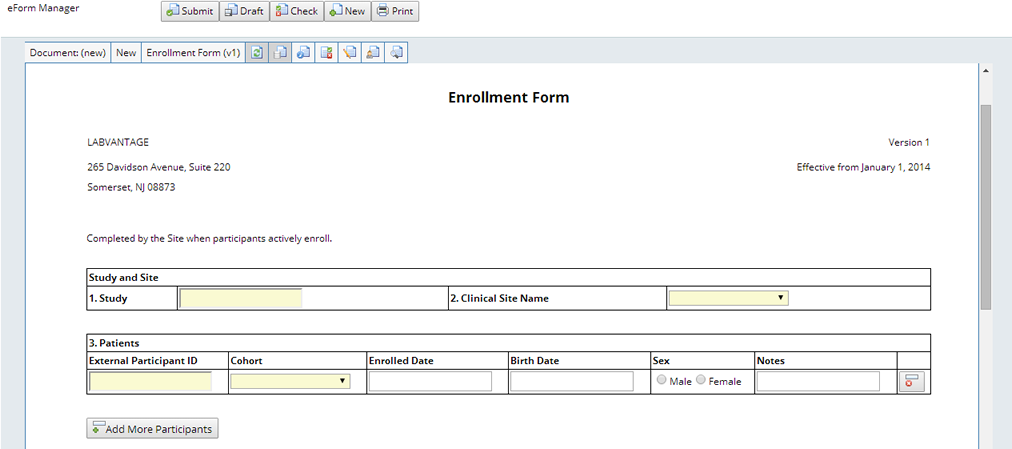
When the new Form is loaded it can be completed and upon submission a new Document is created and processed. The defined processing for the Enrollment Form is to enroll the specified Participants in the current Protocol revision for the selected Study, Site and Cohort:
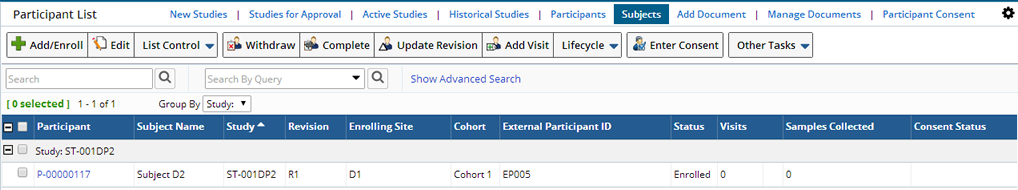
Participant List Page |
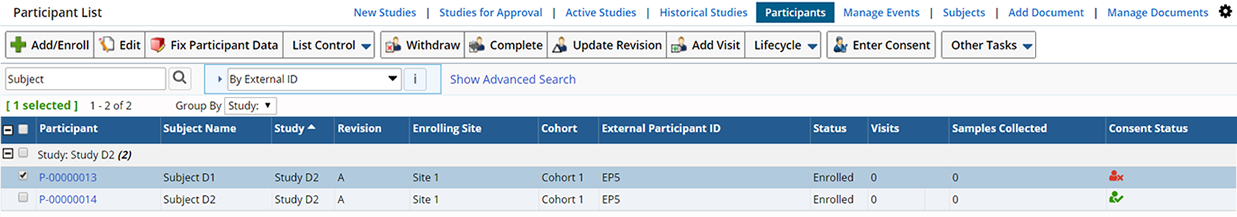
In addition to the standard Add, Edit, View, and Delete operations, the Participant List page allows several other operations to be performed on the selected records:
|
| Column | Description | ||||||||
| Participant | AutoKey-generated identifier of the Participant. | ||||||||
| Subject Name | Name of the Subject enrolled as a Participant. | ||||||||
| Study | Study in which the Participant is enrolled. | ||||||||
| Revision | Protocol revision used for the Participant's Sample collection. | ||||||||
| Enrolling Site | Site to which the Participant belongs for Sample collection for the Study. | ||||||||
| Cohort | Cohort in the Protocol to which the Participant belongs. | ||||||||
| External Participant ID | External identifier used by the Site to identify this Subject in this Study. | ||||||||
| Status | Current status of the Participant in the Study. One of "Enrolled", "Completed", or "Withdrawn". | ||||||||
| Visits | Number of Events associated with this Participant. | ||||||||
| Samples Collected | Number of Samples collected for this Participant in the Study. | ||||||||
| Consent Status | The status of a donor's Consent, based on answers to the Consent Questions defined in the Study.
|
Operations |
| Button | Description | ||||
| Add/Enroll | Opens the Participant Enrollment page to create a new Participant and enroll them in a Study. | ||||
| Edit | Opens the Participant Maintenance page for editing. | ||||
| Fix Participant Data | Opens the Fix Participant page. This lets you correct any errors made during Accessioning.
This button is only available to Users with the "SMS Admin" Role.
The following example shows the Fix Participant page for a Protocol Study.
Depending on the type of Study (Protocol or Non Protocol), and the fields to be changed, as you begin to make changes, related fields are refreshed accordingly (to reflect the Enrolling Sites associated with a different Study for example). As changes are made, be sure to choose the appropriate values in these related fields. Changes to a Participant's External Participant ID are validated against the Sample Family's External Participant ID. This in turn invokes the "Ambiguous Subject Rule" (Biobanking Policy). Changing any data (other than External Participant ID) does not affect any previously accessioned Samples. You will need to manually fix that information on any already collected Samples. Fix Sample Accession DataFrom within the Fix Participant page, click "Fix Sample Accession Data" to correct any Accessioning Data Entry errors. This takes you to the Fix Sample Accession Data Page where you can fix the accessioned data on all collected Samples for the Participant. A warning message is displayed to remind you that changes made here may have a significant impact on other data within the application.
See Biobanking Sample Operations → Fix Sample Data for additional information. |
||||
|
View
(List Control Menu) |
Opens the Participant Maintenance page in read-only mode. Deletes selected Participants. |
||||
| Withdraw (Protocol Studies Only) |
Withdraws the selected Participants from their Studies. Prompts the user to provide a reason why and withdrawal date. If the Protocol defines Forms for the Participant to be created upon Withdrawal, then the necessary Documents associated to the Participant will be created at this time. Sets the Participant status to "Withdrawn" as well as the withdrawal date. | ||||
| Complete (Protocol Studies Only) |
Completes the selected Participants involvement in their Studies. If the Protocol defines Forms for the Participant to be created upon Completion, then the necessary Documents associated to the Participant will be created at this time. Sets the Participant status to "Completed" as well as the completion date. | ||||
| Update Revision (Protocol Studies Only) | Allows the Protocol revision associated with the Participant to be changed during the course of the Study. When a Participant is initially enrolled in a Study it is assigned the Protocol revision currently being used for the Site at which the Participant is attending. At some later time, the Protocol revision might be changed for the Site, but this will not change the Protocol revision for Participants already enrolled at this Site. This button is then used to update the Participant's Protocol revision to the new revision. Updating a Participant's Protocol revision is not required and a Participant can be left at the old revision, or even withdraw from the Study if they do not consent to the new Protocol revision. | ||||
| Add Visit (Protocol Studies Only) |
Opens the Visit Maintenance page to add a new Event to the selected Participant. | ||||
| UnWithdraw UnComplete (Lifecycle Menu) (Protocol Studies Only) |
Undo an incorrect Withdrawal or Complete. | ||||
| Enter Consent | Answer the Consent Questions defined in the Study into which the Participant is being Enrolled. See Enter Consent for detailed information about Consent Questions. |
Participant Maintenance Page |
Using the "Edit" button, from the Participant List page, the Participant Maintenance page opens where only the "External ID" and "Cohort" fields are editable. "External ID" represents the identifier associated with the Participant that is assigned by the Site to which the Participant belongs. If the Study is a Protocol Study the Cohort will default to the Participant, optionally change the Cohort if necessary. All other fields on both the Participant and Subject tabs are read-only:

Primarily the Participant Maintenance page is used to manage the Visits and Documents associated with the Participant.
Click the Fix Participant Data button to correct any errors made during Accessioning. See Fix Participant Data for details.
Participant Visit Maintenance |
From the Visits tab, clicking the "Edit" button allows existing Visits to be edited or Visits can be removed with the "Remove" button. New ad-hoc Visits can also be added using the "Add Visit" button at the top of the page.
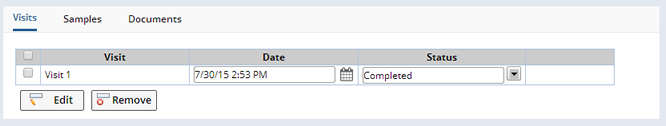
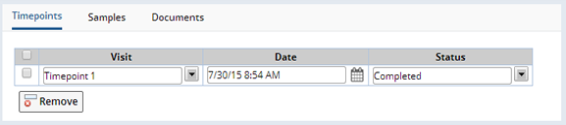
On the Participant Visit Maintenance page, opened from the "Edit" button in the Visits tab above, new Timepoints can be added using the "Add Timepoint" button, or existing Timepoints can be edited or removed:
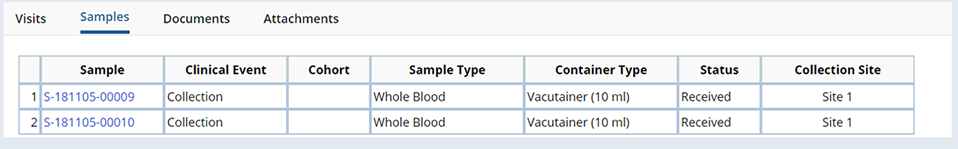
Samples |
Samples associated with the edited Visit, or its Timepoints, are shown on the Samples tab. Click the Sample Id to view Sample details.
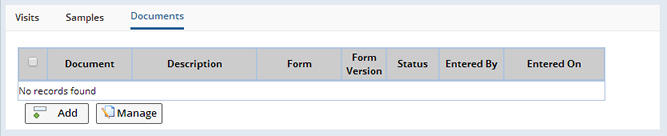
Participant Document Maintenance |
From the Documents tab, new Documents can be added and existing Documents can be managed. Only Documents based on Forms defined in the Protocol as being allowed to be associated with a Participant can be created from this tab. Some of the Documents in the tab may have been automatically created when the Participant was created or its status changed as defined in the Forms for the Protocol.
Upon clicking the "Add" button, the Documents management page opens and allows a new Form to be selected for completion and submission. Example Forms that might be completed from the Participant Maintenance page are the Participant Missed Visit Form, which will create a missed Visit Event record for the Participant, or the Consent information completed by the Participant:
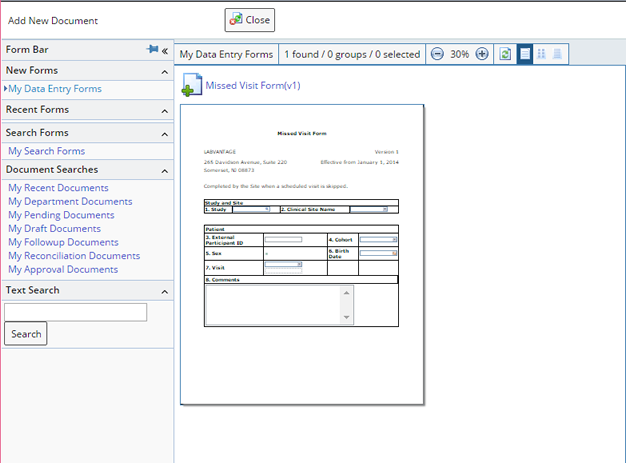
If an existing Document needs to be edited, reviewed or managed, select the document and click the "Manage" button.
The ability to view, add, edit and manage documents in the Participant Maintenance page and the Participant Visit Maintenance page is controlled by the user's role in the Participant's Study as defined in the Study Users tab of the Study Maintenance page.
Participant Event List Page |
Lists all Participant events.
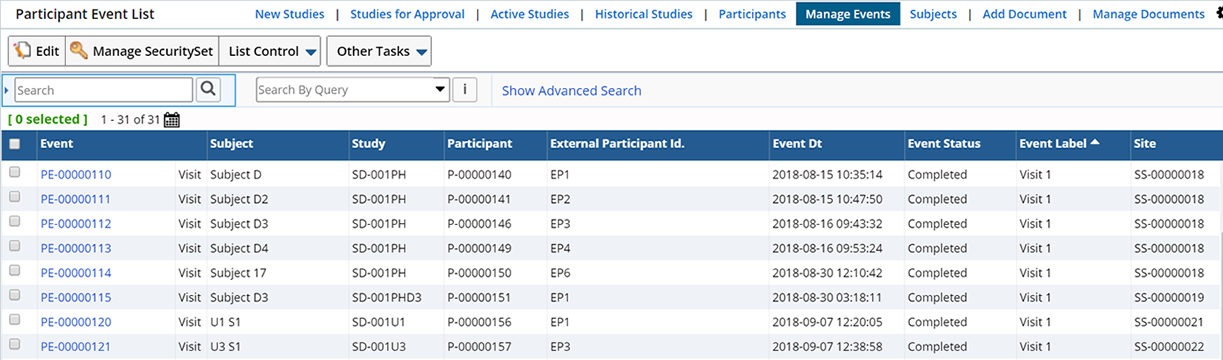
Optionally click the Calendar icon to view these events in Calendar View. Hover over the Event to display Event details.
