Overview
| Top |
|
BioBanking Forms and Documents |
Overview |
|
|
The BioBanking Forms module allows the creation of online web pages with custom layouts that can resemble traditional paper forms. These pages when accessed from the LabVantage application can be completed like a paper form and saved in the database.
LabVantage Forms are reusable containers for the HTML definition of online forms as defined by the W3C. Forms do not have any user data associated with them, but are used as a template for users to complete. Forms define an appearance and layout and can have processing logic and business rules associated with them.
LabVantageDocuments are instances of reusable Form templates. When a Form is accessed, data is then entered into the fields. Upon submitting the Form and its completed data fields to the database, a Document is created and contains the data entered by the user. A Document has a lifecycle that can consist of review and approval steps. In addition the processing logic defined in a Form is executed upon a Document when it reaches the appropriate status based on its review and approval requirements (See eForm Document Reference).
BioBanking Documents can be accessed from the following pages and links:
| • | "Manage Documents" operation on the Study List and Maintenance pages. |
| • | "Manage Documents" button in the Study Site Maintenance tab. |
| • | Documents tab in the Participant Maintenance page. |
| • | Documents tab in the Participant Visit Maintenance page. |
| • | Documents tab in the Subject Maintenance page. |
| • | Documents tab in the Sample Family Maintenance page. |
| • | "Manage Documents" operation on the Sample List pages. |
| • | "Add Document" and "Manage Documents" menu items. |
Form Examples |
|
|
BioBanking Forms can be grouped into two categories:
| 1. | Forms that are designed to provide system functionality. Typically these types of Forms, when processed, would create or update database entities or cause particular actions to take place. Examples of such Forms include Study Participant Enrollment Forms, or Sample Collection Forms. |
| 2. | Forms that are designed to provide additional information. These Forms would include data that augments and annotates standard information. This data could further describe key BioBanking entities such as Samples, Subjects, Participants, or Events without requiring any data model modifications - the data would be stored solely in the LabVantage Document. Examples of such Forms include Participant Consent Forms, or Visit Case Report Forms (CRF). This extended information is searchable by Queries or the Adhoc Query tool and can be used to locate the relevant SDI or to display the additional attributes (see Searching Documents). |
Out of the box examples of BioBanking Forms include:
ParticpntConsent
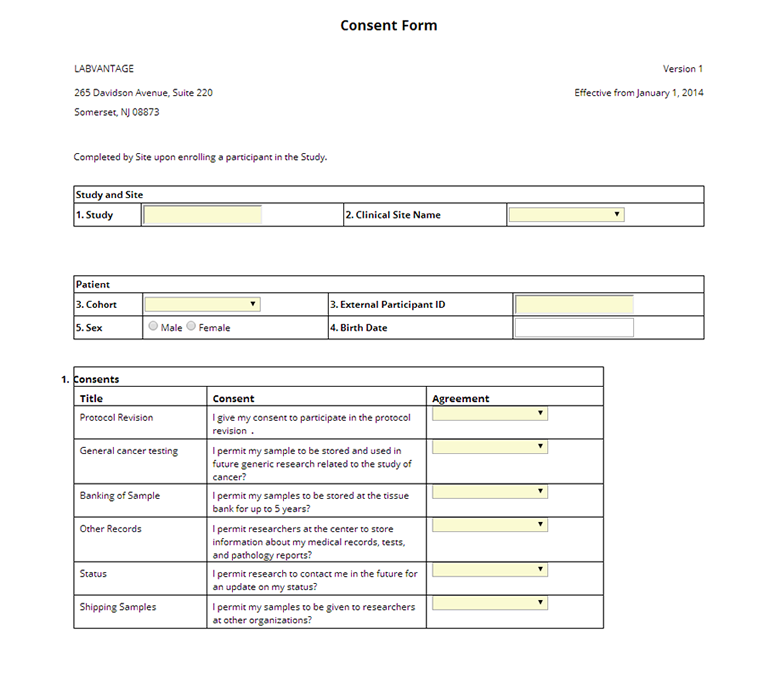
ParticpntEnrollment
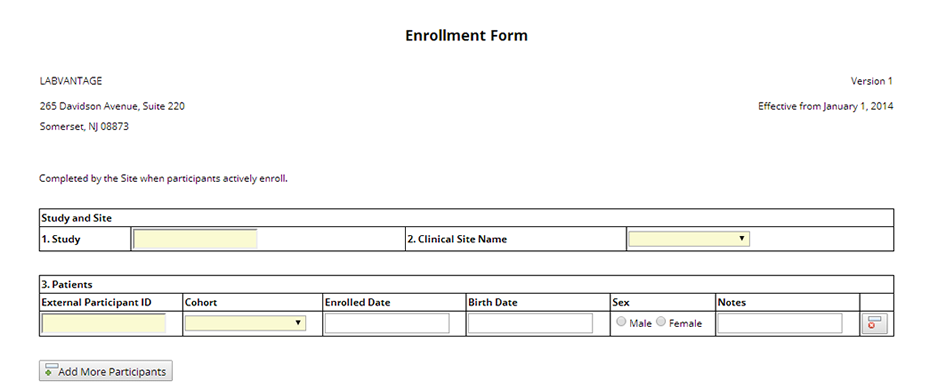
ParticpntMissedVisit
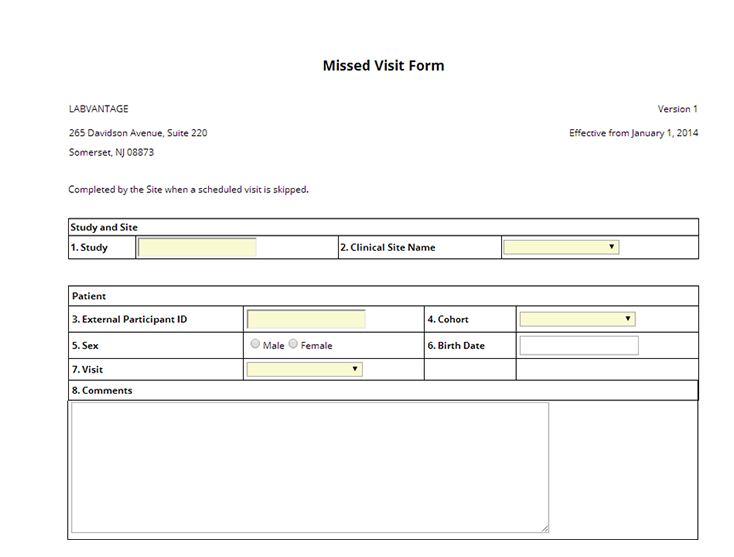
ParticpntVisitCRF
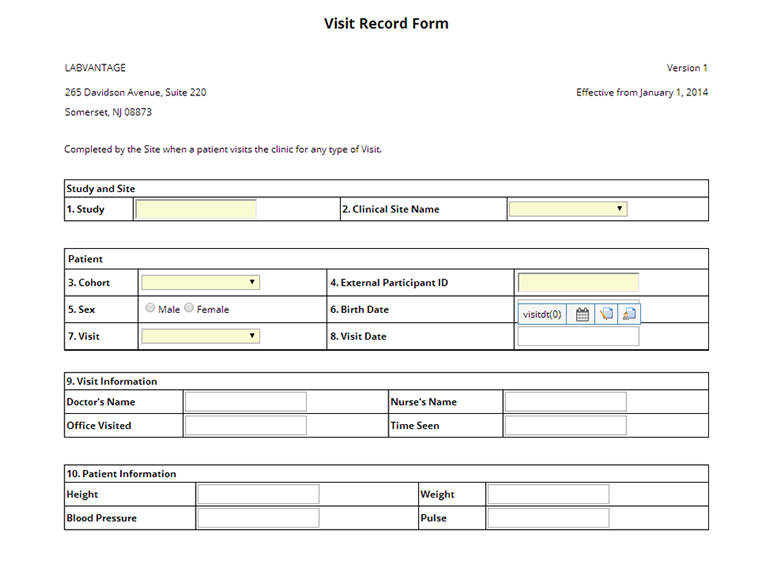
SampleAttributes
SampleCollection

SampleCollectMulti
SampleCollMultiPart
SampleCollMultiTimeP
SubjectHistory
Other Forms can be created and used in the BioBanking module to replicate any paper forms required in the application.
Form Usage in Studies, Protocols and Events |
|
|
Forms can be added to various different levels of BioBanking Studies.
When a Form is defined in a Study, Protocol, or a Protocol Event it is assigned to a particular target SDC as well as to when Documents based on the Form should be created. When an SDI of the chosen SDC is created or modified in some way for the Study, Protocol or Event, it is possible that a blank Document of the specified Form is created and linked to the associated SDI. This Document is then available for completion by the user.
When a Form is defined in a Study, or Protocol as an "on Demand" Form then blank Documents are not created automatically by the system. The Forms in the Study or Protocol simply provide a filtered list of Documents that can be created on an ad-hoc basis by the user for the specified target SDC.
When a Form is defined in a Study, or Protocol and does not specify a target SDC, then these provide a filtered list of Documents that can be created within the context of a Study or Site. This allows the definition of Study specific collection, enrollment, or any other Forms that may be completed within the Study context.
Document Security |
|
|
Security for BioBanking Study Documents is controlled by assigning users a "Document Function" in the Study. This function setting is either for an entire Study or limited to a particular Site within a Study. If a user is not assigned a Document Function in a Study then that user will not be able to access any of the Documents associated with the Study. There are 4 Document Functions defined in the system:
| Document Function | Description |
| DocumentEntry | User can create, edit and view documents. |
| DocumentReconciliation | User can review documents and edit documents to reconcile any errors. Document creation is also possible. |
| DocumentApproval | User can review, edit and approval documents. Document creation is also possible. |
| DocumentManagement | User can perform all operations on Documents. This includes creation, editing, reviewing and approving. |
Searching Documents |
|
|
All data fields in a Document are stored in the LabVantage database and are searchable. This means Document fields can be included as part of a fixed Query, or used in an Adhoc Query.