Introduction |
|
|
QC Batch lets you test a group of Samples (in Batches) at the same time, or in a continuous, sequential order. The Samples, when tested, provide confidence that the analytical process is in control. The process must be in control in order to provide accurate and repeatable results. When not in control, measurements cannot be trusted.
At the heart of AQC is the QCBatch (QCBatch is an ordered set of sdidata objects from different Samples). Samples in the QC Batch are a mixture of the Unknown Sample, along with interspersed QC Samples to assure the process stays in control.
In LabVantage, QC Methods define the planned and systematic actions needed to provide confidence in each analytical result. The QC Batch (based on a QC Method) consists of the different types of Samples (such as Unknowns, Controls or Blanks) and their positioning. These Samples are then evaluated according to a set of applied rules based on the Western Electric Company (WECO) Rules and the Westgard Multi-Rules Procedure.
In general, this is the order in which you use AQC functionality:
Set up the Batch Analysis:
| • | Define QC Rules to specify rules for evaluating the Batch. |
| • | Add a QC Method to define the types of Samples (such as Blanks or Controls) to include in the Batch and the procedures used to process a batch of Samples. Also define any QC Calculations needed for Batch Processing. See QC Calculations for details about the calculations you can define. |
Create and Manage the QC Batch:
| • | Add a QC Batch to identify the Samples for evaluation. See QC Batches for information about managing existing Batches. |
| • | Identify the various Consumable Lots used for the QC Samples. |
| • | Assign the QC Batch to an Analyst or Department. |
| • | Perform QC Batch Data Entry in the order of the QC Batch.
As data is entered, special calculations on the different QC Samples are executed. These calculations calculate properties such as %Recovery, or RelativePercentDifference. These calculated results are then used to evaluate the QCBatch.
Enter data for Preparation datasets and/or Procedural datasets (when Preparation datasets are included preparation data must be entered before procedural data). |
Evaluate and Review the QC Batch:
| • | Evaluate the QC Batch, either manually, or automatically using pre defined QC Rules.
The QCBatch is evaluated in one of two ways: Or, using Control Charts to view trending characteristics across the whole of the QCBatch. These trending checks are defined by the QCEvalRules. Some of the rules can simply check that a result is within a number of standard deviations, or if a result is simply ever increasing in value. The capability to look back at yesterday's qc Batch to get more data points is also provided. |
| • | Once evaluation is complete, Review QC Batch results to determine whether or not the QC Batch is in control. Choose to Re-test if necessary and Publish Parameters to the QC Batch. |
QC Batch Status Transitions |
|
|
QC Batches transition through the following life cycle:
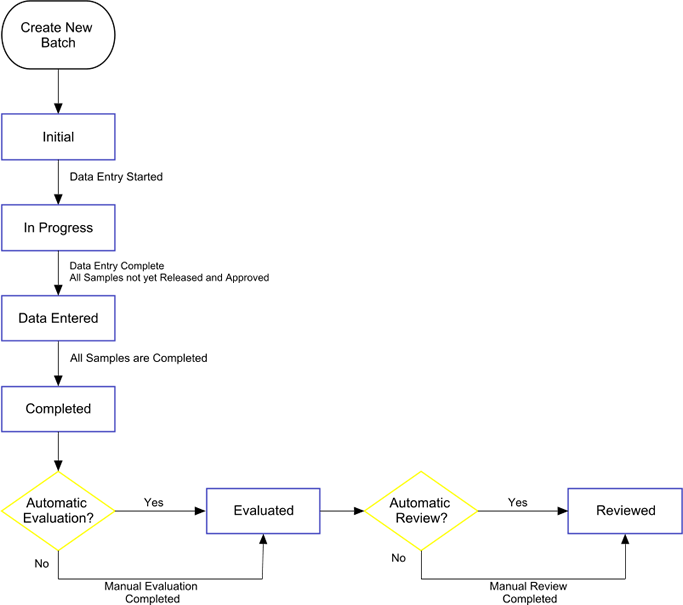
| QC Batch Status Transition | Description | |||
| Initial (create QC Batch) | A new QC Batch has been created. The QC Batch is editable, and a QC Method can be applied. | |||
| Initial → InProgress | Data is being entered for all mandatory Data Items associated with all Samples in the QC Batch. If the status is "InProgress", you cannot modify the QC Batch. | |||
| InProgress → DataEntered | Data have been entered for all Data Items
associated with any defined QC Batch Params (Data Set assigned at the QC Batch
level) and all Sample level parameters.
"Release" and "Approval" of the Sample Level Data Sets have not yet been conducted. |
|||
| DataEntered → Completed | Upon "Release" and "Approval" of associated Test data (datasets) the Status of the relevant Samples in the QC Batch changes to "Completed". Once all are Complete the QC Batch changes to "Completed". | |||
| Completed → Evaluated | The QC Batch has been evaluated, as determined by the "Evaluation Option" defined by the QCMethod. | |||
| Evaluated → Reviewed | QC Batch has been reviewed (automatic or manual review are offered as options). |