Introduction
| Top |
|
BioBanking Overview |
Introduction |
|
|
The Biobanking module provides the ability to design Studies for the purpose of assessing the safety, efficacy and outcomes of a drug, regimen or procedure. Once a Study begins, Biobanking manages Sample Collection, Testing, and Storage. Following is a brief description of the two major functions:
Study design can include a Protocol Study (Include versioned Protocols and Cohorts), or design a Non Protocol Study (define specific Clinical Events). Within the Study, define Collection Events that determine a collection plan including any tests or procedures to perform once the Samples arrive. Generate Collection Kits for collecting the Samples needed for the Study. Define Consent Questions to identify any restrictions on a Sample's use.
Sample Management tracks and manages Samples once they arrive at the facility. Sample Management provides the following functionality:
| • | Receive and Accession expected Samples as well as enroll the Subjects (Sample donor) as Participants in the Study. |
| • | Services manage any tests and procedures to be performed on the Samples and creates any additional Child Samples needed for testing. |
| • | Manage the Storage of Samples while in the facility including any Freeze Thaw requirements. |
| • | As needed Users can move Samples between Custodial Departments according to defined rules. |
| • | Samples can be Packaged and Shipped to Internal or External Shipping Locations Management also manages the shipment and storage of Samples. |
| • | Search for and Pull Samples according to Requests submitted to the facility. |
Study Life Cycle |
|
|
A Study groups a collection of Samples under a specific experimental premise or clinical protocol. Generally studies are a set of Samples collected loosely from different sources that are to be used for a common purpose such as general discovery science.
The following diagram illustrates the Life Cycle of a Biobanking Study including a Protocol.
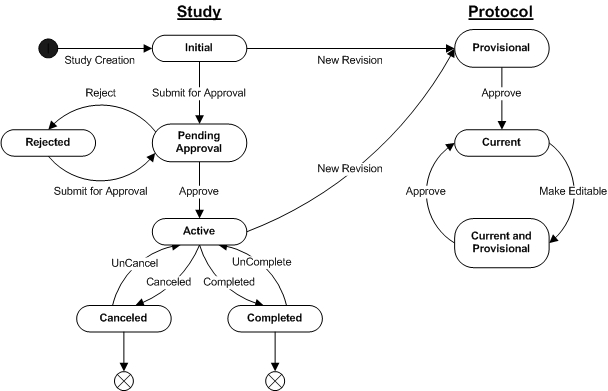
Study State Descriptions |
| State | Description |
| Initial | Study state when it is initially created. |
| Pending Approval | Study details have been completed and it is ready for approval. |
| Active | Study has been approved and is now in active use. |
| Rejected | Study has been rejected and needs to be edited and resubmitted for approval. |
| Completed | Study has been completed and is no longer in use. |
| Canceled | Study has been canceled for some reason and is not in use. |
Study State Transitions |
| State | Description |
| Study Creation | Initial information for the Study
is completed by the user and saved. |
| Submit for Approval | All details of the Study,
including the Protocol are completed, saved and then submitted for
approval. |
| Approve | The necessary users have reviewed
and accepted the Study for approval. |
| Reject | One or more of the approval users, according to the approval types, have rejected the Study for approval. |
| Completed | Study is marked as no longer
active by the user. |
| UnComplete | Study is restored to an active state when incorrectly completed. |
| Canceled | Study is canceled by the user. |
| UnCancel | Study is restored to an active state when incorrectly canceled. |
Protocol State Descriptions |
| State | Description |
| Provisional | Protocol state when a new revision
is initially created. |
| Current | Protocol is available
for use by the Study. |
| Current and Provisional | Current Protocol has been
edited and a new version is created. |
Protocol State Transitions |
| State1 | Description |
| New Revision | From Study maintenance,
the user requests a new
revision of a Protocol, that is then created with the initial
information supplied by the user. If a Protocol already exists and is
selected, then details of the selected Protocol are copied to the new
revision as a starting point for changes. |
| Approve | The Protocol is reviewed
and
approved by the user. |
| Make Editable | Upon a user request an existing
approved Protocol is made editable by creating a new version of
the selected Protocol revision. |
| 1 | The Protocol version status is not shown for simplicity. When a Protocol Revision is first created, it has a version status of "Provisional". Once the Protocol is approved, its version status becomes "Current". When a new version of a Protocol Revision is created, by making the current approved version of the Protocol Revision editable, the new version also has a version status of "Provisional". Once the new version is approved, its version status now becomes "Current" and the prior current version has its version status changed to "Active". |
Sample Management Life Cycle |
|
|
Parts of the sample lifecycle (such as Sample Collection) may occur outside of the system and do not require any interaction with LabVantage. Collection Events defined in the Study detail those expected Samples. Samples are collected according to the Study, then sent to a facility where they are managed by LabVantage. Samples tracked within the system have a lifecycle that describes a Sample from its initial allocation to its final disposition. This lifecycle contains steps required to properly maintain Samples available to researchers (in circulation), and is not necessarily related to their location in the lab. Therefore, the sample lifecycle is mainly orthogonal to transfers of custody, location management, and sample data requirements. However, the lifecycle defines when a Sample comes into existence, and when it is no longer expected to be tracked.
The Sample lifecycle fulfills the following purposes within the requirements:
| • | Defines when a sample comes into physical existence. |
| • | Specifies operations that must occur to new Samples prior to their availability. |
| • | Ensures Sample data is properly entered. |
| • | Defines the point at which the Sample ceases to exist, i.e., becomes disposed. |
Throughout the system's custody-related operations, the lifecycle state is consulted in order to establish if the sample exists, or can be used for a particular operation. These validations are repeated in the functional description for each feature.
The sample's lifecycle is modeled using sample status values that indicate a Sample's stage in the lifecycle.
At a high level, the sample lifecycle defines the following general steps:
| • | Allocation A notification to the system that a material may arrive at some point and a bar code must be printed for that material. |
| • | Collection and Labeling The association of a physical material with a bar code label or other identifier. |
| • | Accessioning The process of initially receiving a material, entering phenotypic information, and certifying the sample for release to circulation. |
| • | In Circulation Indicates that the material is available for use by anyone (subject to Sample restrictions). |
The general steps can be further defined by these sample lifecycle statuses:
| Step | Description |
| Allocation | A sample ID is created, printed onto a bar code, and the barcode is shipped to a collecting facility. |
| Sample Collection | Outside of the system, a barcode is placed on a biological specimen and, in some cases, a "specimen collection form" is produced. This step does not necessarily require any handling within the system. |
| Move to In Lab Status | This is optional. In some cases, Samples are not shipped directly to a Repository... they may be shipped directly to a laboratory. In that case, the laboratory must record that they have received the Sample (confirming the physical existence of the Sample.) The remainder of the Sample and its Aliquots will always travel to a Repository as their next stop. |
| Sample Receipt | The Sample arrives at a Repository and is formally "accessioned" into the system. Critical information about the source of the material is entered, and users can assign a storage location for the Sample. |
| Data Entry | Newly accessioned samples must receive additional information collected on their "Sample Collection Form." At this point, the donor of the material is registered in the system and associated with the Sample. |
| Additional Verification | This is optional. In some cases, Samples may also travel to a separate group or entity to verify or enter additional information about the specimen. |
| Release to Inventory | After data entry and optional verification are complete, the Sample formally becomes available for request. It is considered "In Circulation. |
| Distributed Samples | Samples within a lab may be transferred to other labs, or back to the Repository. |
| Child Sample Creation | Any Aliquot or Derivative product of a Sample can be recorded within the system as a separate Sample. This is a two-step process. First, the Aliquot is registered, allocating it a new identifier in the system. Second, the Sample is "confirmed", indicating that the Aliquot was actually produced. |
| Disposition | A variety of factors may cause a Sample to leave "circulation." These dispositions include destruction, consumption, and missing. Users record all Sample dispositions. |
| Archiving | A Sample may be removed from circulation by storage for an indefinite period of time. |
| 3rd Party Transfer | A Sample may be transferred beyond the facilities' responsibility. |
The following diagram shows Sample status values and transitions from one status to another:

Sample State Descriptions |
| State | Description |
| Allocated | Sample is a "virtual" entity that may or may
not physically exist in the laboratory.
This assigns an identifier to the Sample before it arrives in the laboratory. |
| Received | Sample no longer "virtual"... it is physically received into the laboratory and officially exists in the lab. However, it is not yet available for use within the laboratory. |
| Verification Needed | Authorization or verification of Sample data is required prior to being put "In Circulation". |
| In Circulation | Sample is available for use within the laboratory. |
| In Prep | If a child Sample, Sample is prepped prior to being made available for use with the laboratory. |
| 3rd Party Transfer | Sample is transferred beyond the control of the laboratory. |
| Archived | Sample is no longer available for use within the laboratory, but it still physically exists in the laboratory. |
| Disposed | Sample no longer physically exists within the laboratory (consumed, intentionally destroyed, or lost). |
Sample State Transitions |
| State | Description |
| Allocation | Identifier is assigned to the Sample. |
| Receive | Sample is physically received into the laboratory. |
| Approval | Sample data are approved. |
| Conditional Approval | Sample data are approved, with the requirement that final approval is pending. |
| Request Additional Verification | For a Study, an additional party is requested to verify Sample data. |
| Complete Additional Verification | For a Study, the additional party verifies Sample data. |
| Child Sample Creation | An Aliquot, Derivative, or Pooled Sample is created. |
| Child Sample Confirmation | Details regarding the child Sample are confirmed. |
| 3rd Party Transfer | Sample is transferred beyond the control of the laboratory. |
| Archive | Sample is taken out of use within the laboratory. |
| Make Available | Sample that was taken out of use within the laboratory is returned to use. |
| Dispose | Sample no longer exists within the laboratory. |
Master Data Setup |
|
|
Before using Biobanking consider defining the following Master Data items:
Define these system-level attributes:
| • | Reference Types to identify:
|
||
| • | Parameter Lists for use when defining Services. | ||
| • | Data Masking lets you protect sensitive information from unauthorized Users. |
Define Laboratory level information that will be used when creating Studies.
| • | Contacts, internal or external contacts, LabVantage Users |
| • | Products, used when defining Protocol Studies. |
| • | Storage Environments such as Hot or Cold |
| • | Containers to be used throughout Studies |
| • | Create Biobanking Forms used to collect information such as Subject enrollment details, Subject consent information, Site material transfer agreements (MTA) or details of Samples collected. Define templates of these documents as online Forms that can then be completed to create Documents within LabVantage. |
Define the following Biobanking Specific Master Data:
| • | Services to define the laboratory operations that need o be performed on incoming Samples. |
| • | Study Templates provide default information about the different types of Studies you will create. Reference Study Templates when creating new Studies. |
| • | Kit Types define the different types of Collection Kits you might create for collecting Samples. |
| • | Collection Methods to define the types of methods used to collect Samples. |
| • | Define different Preparation Types such as Blood Prep or slice Tissue Block. |
| • | Specify any Treatments such as Master Mix or Dye. |
| • | Determine the Species of expected Samples. |
| • | Define the Assay Types you might perform. |
| • | Create Child Sample Plans for creating Child Samples from Received Samples. |
See Biobanking Master Data Setup for more detailed information about Biobanking specific Master Data.
Biobanking Navigation |
|
|
Access Biobanking operations by navigating to:
| • | Master Data from Lab Admin → Biobanking |
| • | Study Design and Management from LIMS → Biobanking |
| • | Sample Management from LIMS → Sample Management |